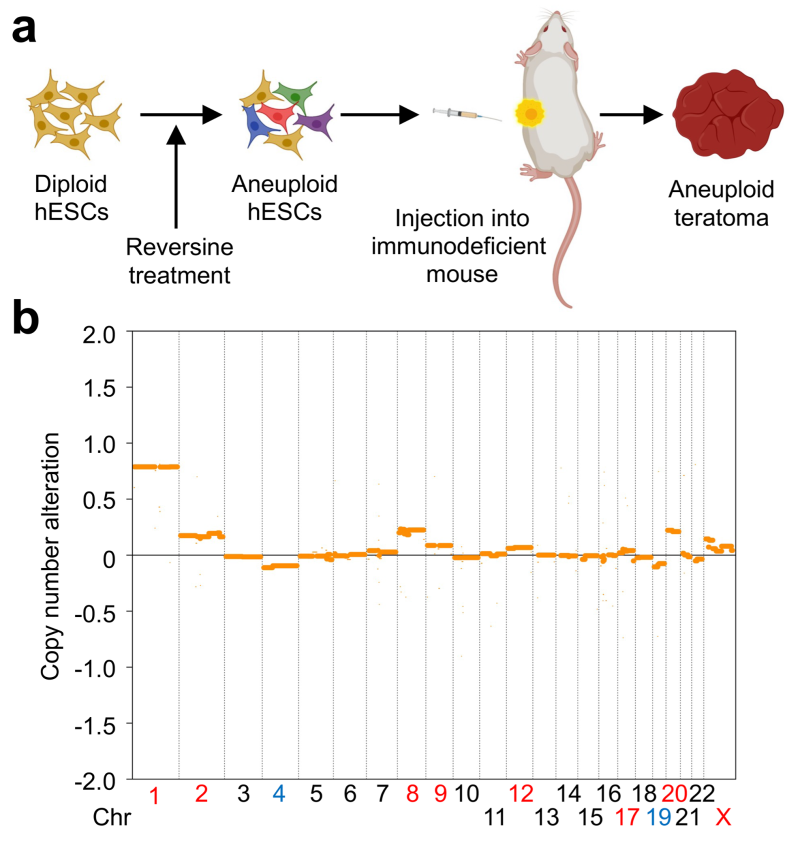
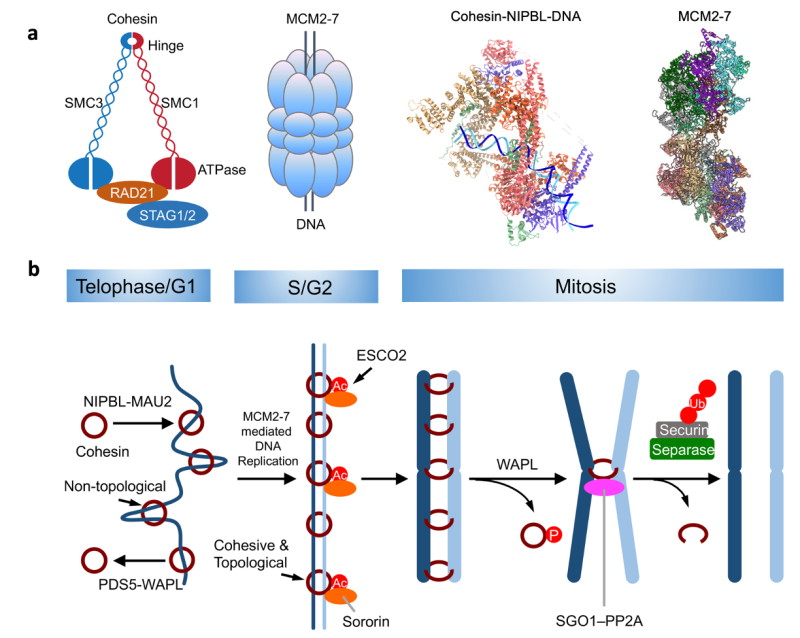
The diploid human genome consists of 6 billion nucleotides, which are assembled into 22 pairs of autosomes and 2 sex chromosomes. Chromosomes in a single human diploid cell, if linearly stitched together, span a length of more than 2 meters. They need to be folded to be housed in the cell nucleus with a diameter of about 10 µm. Chromosome folding occurs in a dynamic, structured way that regulates gene expression, and DNA replication and repair.
There are about 200 cell types in the human body. All have the same genome. It is the selective expression of specific subsets of genes in pluripotent stem cells that determines the cell fate. We hypothesize that dynamic 3D genome organization makes and breaks long-range interactions between distal DNA elements, including promoters and enhancers. As such, it shapes the gene expression landscape of the genome in response to developmental cues, thereby impacting cell fate decisions. Consistent with this hypothesis, we have recently shown that deletion of the cohesin subunit gene STAG2 in the nervous system causes delayed differentiation of the oligodendrocyte lineage, hypomyelination, and neurological defects.
We will further test this hypothesis using brain organoids derived from hESCs as the model system. We have successfully cultured human cerebral organoids from hESCs that have the expected cell types (e.g. neural progenitors, neurons, and oligodendrocytes) and tissue patterning (e.g. neural rosettes). In the future, we hope to establish the mechanism by which cohesin-mediated 3D genome organization regulates gene expression and cell fate decisions.